Why is graphite soft and diamond hard?
When writing with a pencil, sheets of graphite slide off and deposit on the paper. But upon pressing a diamond scribe onto a glass slide, scratches are carved into the glass surface. Why is graphite soft and diamond hard? They are both made solely from carbon atoms, and thus we know that chemical composition is not the cause for the difference in properties. Instead, the difference arises from how the atoms are arranged and held together through chemical bonds. The atoms do not form isolated molecules, but instead have bonding that extends throughout the entire material in what is called a crystal structure. Graphite has layers of carbon atoms, and while the bonding within an individual layer is covalent and strong, the bonding between layers is of the weak Van der Waals type (Figure 1). As a result, the layers easily detach from each other and flake off. On the other hand, diamond has a three-dimensional network of strong covalent bonding (Figure 2). It is one of the hardest known materials and is adhered to the tips of saw blades to aid in cutting. The two forms of carbon are termed phases because they differ in structure. Diamond forms under higher pressure and temperature than graphite and thus is denser and has more chemical bonds. Atoms cannot be seen with the naked eye, and scientists instead use x-ray diffraction to understand the crystal structure of materials.
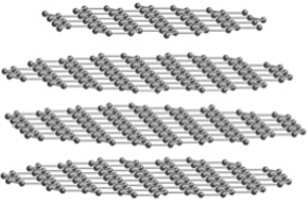

Figure 1: The crystal structure of graphite. A pencil with a core of graphite surrounded by wood.
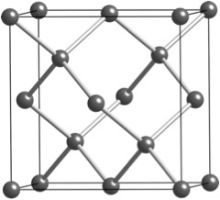

Figure 2: The crystal structure of diamond. The tip of a saw blade with small diamond crystals on its surface
Why is steel strong?
Many materials are more complex and are composed of more than one type of chemical element. While these materials may appear homogenous to the eye, at the microscopic level there are regions of different composition and crystal structure, called phases. Steel is one such material and is a very important component of many things such as buildings and machines. It is made mostly of iron, but contains ~1% carbon that aggregates in regions of cementite whose composition has a ratio of three iron to one carbon. The iron crystallizes in the ferrite structure, while the cementite has its own different structure. Thus, steel has two different phases that coexist with a spatial arrangement that is known as a microstructure. The two phases differ in both chemical composition and crystal structure, and thus have significantly different properties. The ferrite is soft and ductile, whereas the cementite is hard and brittle. The steel is first homogenized at high temperature and then is cooled quickly down to room temperature. Following this, it has a microstructure called pearlite, which is defined by a pattern of alternating ferrite and cementite layers (Figure 3). The steel has an increased mechanical strength because of both the presence of the two phases and because of their pearlite microstructure, however this comes at the cost of reduced ductility. Annealing, or heating the steel at moderate temperatures for an extended period of time, causes the cementite to form spherical regions inside the ferrite matrix. This new microstructure is called spheroidite and the reduced cementite connectivity results in a lower strength but restores much of the ductility. The type of microstructure that forms is also affected by how much carbon is added to the iron because this affects the relative amounts of the ferrite and cementite phases. The microstructure cannot be seen with the naked eye, so scientists instead must use an optical microscope to take pictures of the steel.
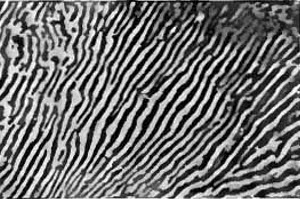
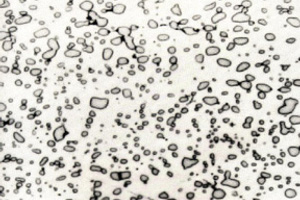
Figure 3: Pearlite and spheroidite microstructures of steel. The light regions are ferrite while the dark are cementite.